

At the 16th Shanghai International Breast Cancer Symposium held during October 21-23, 2021, two studies led by Director Wang Kun of the Breast Oncology Department, Guangdong Provincial People’s Hospital were chosen as 2021 important clinical studies on breast cancer of China. The findings of these two studies were reported by Prof. Wu Jiong, Director of the Breast Cancer Committee, China Anti-Cancer Association, at China’s largest specialized conference on breast cancer and Shanghai International Breast Cancer Symposium.

The NeoCART study (NCT: 03154749) is the first prospective, multicenter, randomized controlled phase II clinical trial that compares 6-cycle docetaxel plus carboplatin (DCb) and standard 4-cycle EC (epirubicin + cyclophosphamide) sequential 4-cycle docetaxel in a neoadjuvant chemotherapy for triple-negative breast cancer (TNBC). This study was led by Prof. Wang Kun of Guangdong Provincial People’s Hospital, and the included patients were from six research centers of Guangdong Provincial People’s Hospital, Shantou Central Hospital, the First Affiliated Hospital of Sun Yat-sen University, Henan Cancer Hospital, Dongguan People’s Hospital, and Baotou Cancer Hospital. The overall pCR rate of the 6-cycle DCb (docetaxel + carboplatin) program of the test group was 61.4% (95% CI 47.0 - 75.8), higher than 38.6% (95% CI 24.3 - 53.0) of 4EC-4D in the control group (odds ratio 2.52, 95% CI 2.4 - 43.1; p = 0.033), with the absolute value increasing by 22.8%. In the analysis of adverse reactions, the use of carboplatin led to greater blood platelet reduction, but focused on 1-2 degrees; while greater neutrophil reduction occurred in the 4EC-4D group.
The NeoCART study provided new evidence and a new treatment option for the neoadjuvant therapy of TNBC, and the high pCR rate indicates good long-term survival. The preliminary results of this study were released at the 56th annual meeting of the American Society of Clinical Oncology (ASCO 2020), and the research paper titled “Neoadjuvant docetaxel plus carboplatin vs. epirubicin plus cyclophosphamide followed by docetaxel in triple-negative, early-stage breast cancer (NeoCART): Results from a multicenter, randomized controlled, open-label phase II trial” was formally published on International Journal of Cancer in September 2021.

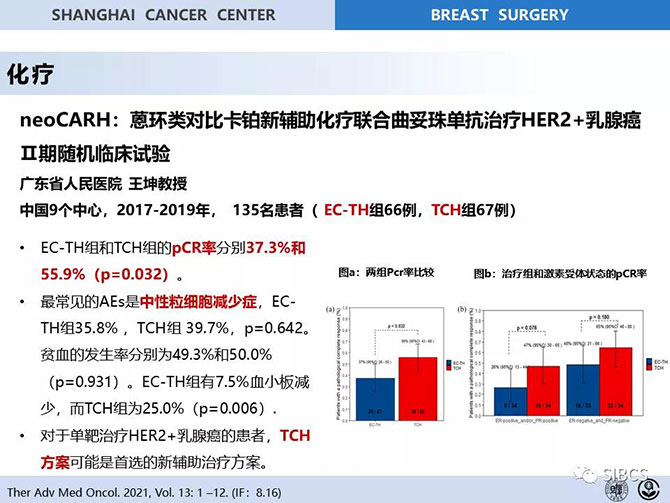
The NeoCARH study (NCT03140553) is the first prospective, multicenter, randomized controlled phase II clinical trial that compares the neoadjuvant chemotherapy 6-cycle docetaxel, carboplatin plus herceptin (TCH) and standard 4-cycle EC (epirubicin + cyclophosphamide) sequential 4-cycle TH (docetaxel + herceptin) in operable HER2 positive breast cancer. This study was led by Prof. Wang Kun of Guangdong Provincial People’s Hospital, and the included patients were from ten research centers of Guangdong Provincial People’s Hospital, Shantou Central Hospital, First Affiliated Hospital of Sun Yat-sen University, Zhejiang Provincial People’s Hospital, Dongguan People’s Hospital, Guangdong Provincial TCM Hospital, Henan Cancer Hospital, Jiangxi Provincial Cancer Hospital, the First Affiliated Hospital of Zhejiang University, and Baotou Cancer Hospital. From May 2017 to the end of the study in November 2019, among 140 randomized patients, 135 were included in this study. The overall pCR rate of an experimental TCH (docetaxel + carboplatin + herceptin) program for six cycles group was 55.9% (38/68) (95% CI, 43.3–67.9), significantly higher than 37.3% (25/67) (95% CI 25.8–50.0) (p=0.032) of 4EC-4TH (an epirubicin plus cyclophosphamide for four cycles followed by docetaxel plus herceptin for four cycles group) of the control group. In the analysis of adverse reactions, 4EC-4TH has a higher proportion of neutrophil reduction, while the 6TCH group had higher proportions of anemia and blood platelet reduction. This was the first prospective randomized controlled phase II trial, and a clinical study on the head-to-head comparison of 6TCH and 4EC-4TH as a neoadjuvant therapy for HER2 positive breast cancer. This study found no statistical difference in adverse reaction rate between the two groups, but the 6TCH group had a higher pCR rate. This research finding proposed to apply a targeted therapy to the neoadjuvant stage of HER2 positive breast cancer to further increase pCR rate, which could change the current neoadjuvant therapy strategy for HER2 positive early-stage breast cancer. This research finding was first reported by Prof. Wang Kun as invited at the annual meeting of the Chinese Society of Clinical Oncology – the largest academic conference of China in this field - in September 2019, included by the annual meeting of the American Society of Clinical Oncology – the largest academic conference of the world in this field – as an abstract and displayed to the public in 2020, and then published on Therapeutic Advances in Medical Oncology in April 2021!
The team of Prof. Wang Kun at the Breast Oncology Department of our hospital has conducted a series of fundamental, translational and clinical studies in the field of the neoadjuvant therapy of breast cancer. Not only have their research findings been recognized by the domestic and global academic communities, but also changed the clinical practice and guidelines through clinical research, and brought the hope of healing to early-stage breast cancer patients.
Zhang Liulu, Gao Hongfei