

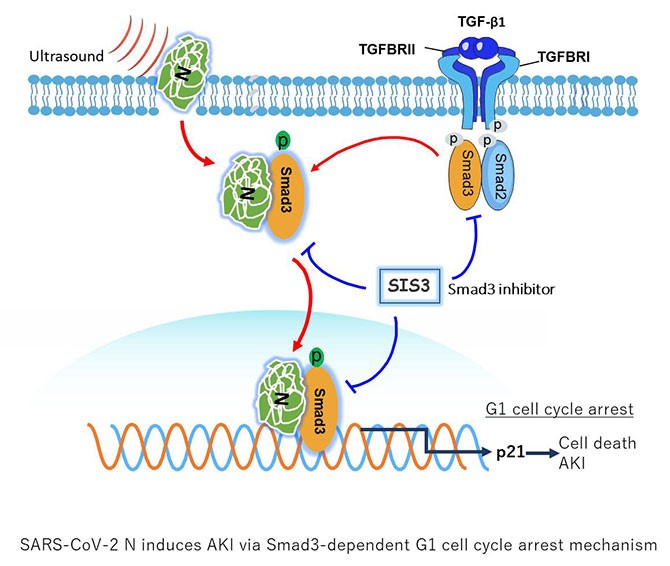
Recently, Prof. Lan Huiyao's team from the Department of Pathology of Guangdong Provincial People's Hospital published an article entitled “SARS-CoV-2 N protein induces acute kidney injury via Smad3-dependent G1 cell cycle arrest mechanism” (DOI: 10.1002/advs.202103248) in Advanced Science. This article is the first to demonstrate that SARS-CoV-2 N protein can induce acute kidney injury (AKI) by using the ultrasound-microbubble technique. SARS-CoV-2 N protein can interact with Smad3, a downstream signaling molecule of TGF-β1, and cause tubular epithelial cell death and AKI via the G1 cell cycle arrest mechanism.
Acute kidney injury (AKI) is common in critically ill COVID-19 patients, characterized by elevated serum levels of creatinine and tubular necrosis. According to published data, the incidence of AKI in critically ill COVID-19 inpatients exceeds 40%, with a significantly higher mortality rate. It has also been shown that AKI is the second highest complication in critically ill COVID-19 patients after the acute respiratory failure. Therefore, it is crucial to uncover the specific mechanisms of SARS-CoV-2-induced AKI.
By using the ultrasound-microbubble technique, the research team found that SARS-CoV-2 N protein could directly induce AKI by kidney-specifically overexpressing SARS-CoV-2 N protein in normal mice, characterized by tubular necrosis and elevated serum levels of creatinine. The symptom worsened in mice with ischemic reperfusion injury (IRI), which was consistent with published clinical data.
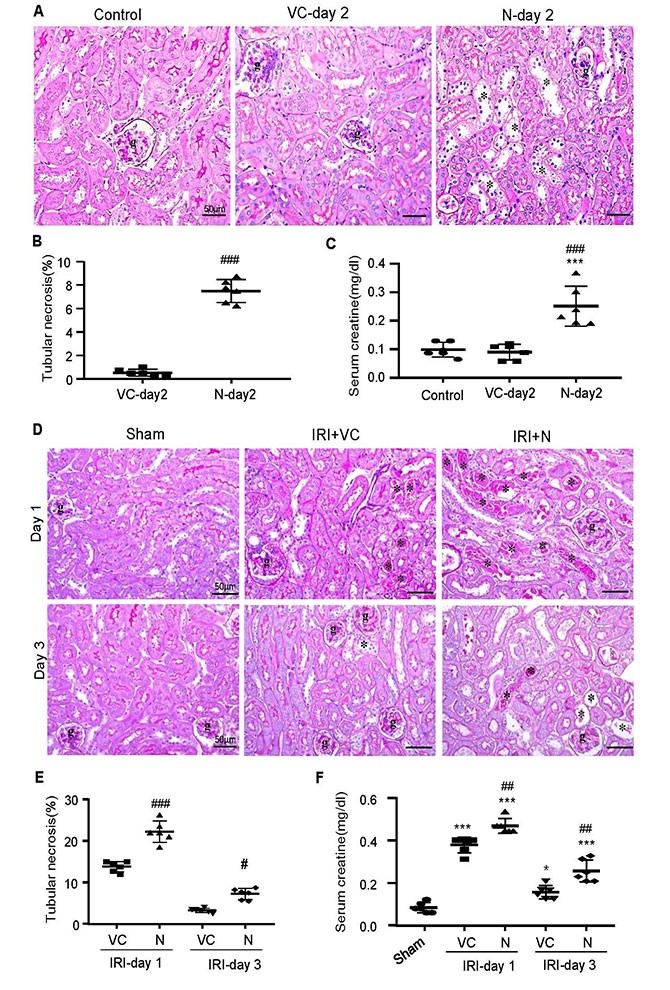
Ultrasound-microbubble-mediated kidney-specifically overexpressing SARS-CoV-2 N protein can induce AKI and make it become worse.
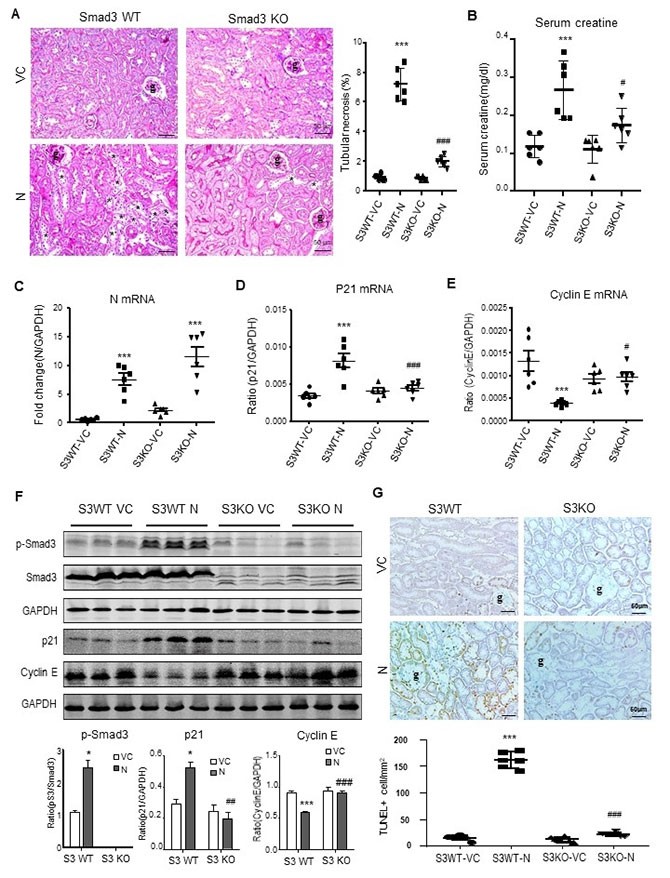
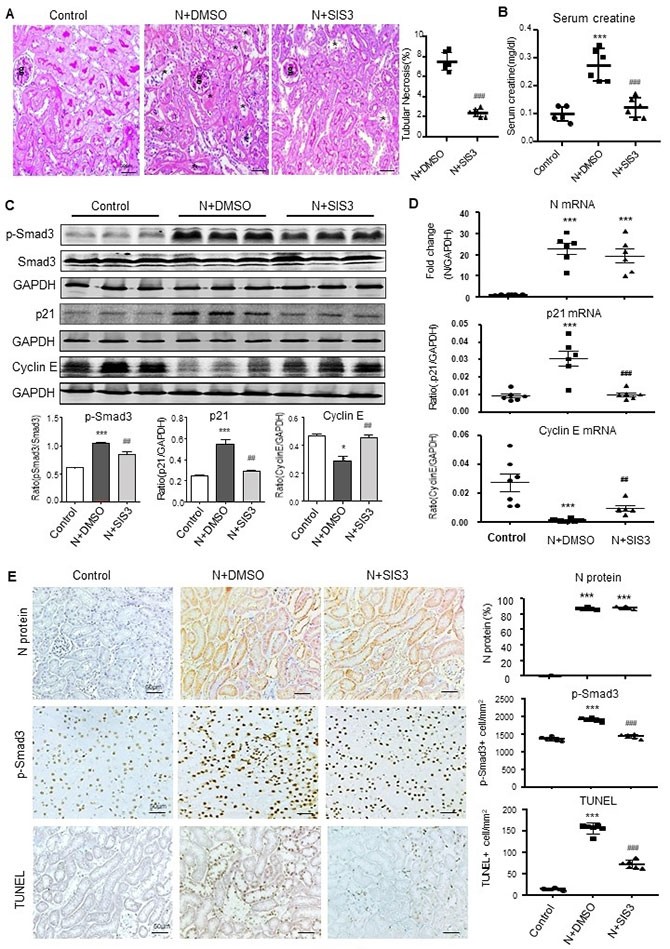
Further studies revealed that SARS-CoV-2 N protein may trigger activation of Smad3 signaling to cause cell death and induce AKI. By studying Smad3 knockout mice and animals with specific Smad3 inhibitors, they found that the deletion of Smad3 gene and the inhibition of Smad3 phosphorylation significantly protects against SARS-CoV-2 N protein-induced cell death and AKI, suggesting that Smad3 could be a new drug target for the treatment of SARS-CoV-2 N-induced AKI.
SARS-CoV-2 N-induced AKI is Smad3-dependent.
Smad3 inhibitor (SIS3) can inhibit SARS-CoV-2 N protein-induced AKI.
Prof. Lan Huiyao and Prof. Yu Xueqing of Guangdong Provincial People’s Hospital and Prof. Wu Jianguo of Jinan University are the corresponding and co-corresponding authors of the paper. Dr. Wang Wenbiao, introduced talent of GDPH, and Dr. Chen Junzhe of Southern Medical University are the first and co-first authors of the paper. The study was supported by the Research Grants Council of Hong Kong (14101121), the Guangdong-Hong Kong-Macao-Joint Labs Program from Guangdong Science and Technology (2019B121205005), the Lui Che Woo Institute of Innovative Medicine (CARE program), the Hong Kong Scholar Program (XJ2019052), and the National Natural Science Foundation of China (81902053).
Prof. Lan Huiyao was introduced by GDPH Department of Pathology in 2019 via the flexible introduction of talents program. He is currently the associate dean of the Faculty of Medicine, Chinese University of Hong Kong, associate director of the Li Ka Shing Institute of Health Sciences, director of the Laboratory of Inflammatory Diseases, senior chairman of the Hong Kong Association for the Advancement of Science and Technology, associate director of the 2nd Academic Committee of Asia-Pacific Association of Medicine and Biological Immunology, associate director of the Asia-Pacific Association of Fundamental Immunology, vice president of the Immunology Professional Committee of the World Federation of Chinese Medicine Societies, health expert and consultant of Guangdong-Hong Kong-Macau Greater Bay Area. His specialized research area is the role of TGF-β/Smad signaling in immune-mediated inflammatory diseases (including hypertension, diabetes-related cardiovascular and renal diseases, and tumor microenvironment). He is in charge of more than 70 research grants, such as NIH of America, NH&MRC of Australia, RGC of Hong Kong, HMRF, ITF, etc. He has published 365 SCI articles, with >35,000 citations and an h-index of 104.
Text: Wang Wenbiao, Photo: Chen Junzhe