

On September 8, 2022, under the concerted efforts of the whole team, the paper titled “Ultrasound-assisted Carbon Nanoparticle suspension mapping Versus Dual Tracer-guided Sentinel Lymph Node Biopsy in Patients with Early Breast Cancer (UltraCars): Phase Ⅲ Randomized Clinical Trial” was published online in the British Journal of Surgery(IF=11.12), one of the three top surgery journals. Professor Wang Kun from the Breast Oncology Department of our hospital is the corresponding author. Dr. Zhang Liulu and Dr. Cheng Minyi are co-first authors.
Breast cancer has the highest incidence among malignant tumors in the world and is the leading cause of death in women with cancer. The conventional surgery is to dissect all axillary lymph nodes, but large-scale dissection surgery can bring about a series of complications such as restricted shoulder movement and sensory dysfunction. Sentinel lymph node biopsy is the standard axillary treatment of early clinically-negative axillary breast cancer and can exempt some node-negative patients from axillary dissection and reduce surgery-related trauma. For a long time, the standard tracer method for sentinel lymph nodes is a dual-tracer method of combined dye-radioactive tracer technique, but it is difficult to apply it in China due to radionuclide contamination.
In the early retrospective study, the team led by Professor Wang Kun explored the feasibility of applying the carbon nanoparticle suspension developed independently by our country in sentinel lymph node biopsy. The detection rate was as high as 99.1% and the false negative rate was 4.1%. The paper titled “Application of a Carbon Nanoparticle Suspension for Sentinel Lymph Node Mapping in Patients with Early Breast Cancer: A Retrospective Cohort Study" was included in the Clinical Practice Guidelines for Sentinel Lymph Node Biopsy in Patients with Early-stage Breast Cancer: Chinese Society of Breast Surgery (CSBrS) Practice Guidelines.
The subsequent UltraCars study was an open-label, prospective, randomized controlled Phase Ⅲ clinical study. The test group was an intraoperative ultrasound-assisted carbon nanoparticle tracer sentinel node biopsy and the control group was a standard dual tracer. A total of 330 patients were enrolled in the study. The detection rate of the test group was 94.5% (95% CI: 90.9–98.0) which was comparable to the detection rate (95.8%) of the dual tracer group. In addition, there was no difference in lymph node metastasis rate or a median number of lymph nodes detected. The mean surgery time of intraoperative ultrasound-assisted carbon nanoparticle tracer sentinel node biopsy surgery was 7.53±2.77 min, which was not longer than the dual tracer group (P=0.316). In addition, the detection rate of the test group in the subgroup after neoadjuvant therapy was still as high as 91.7% (95% CI: 82.2–100).
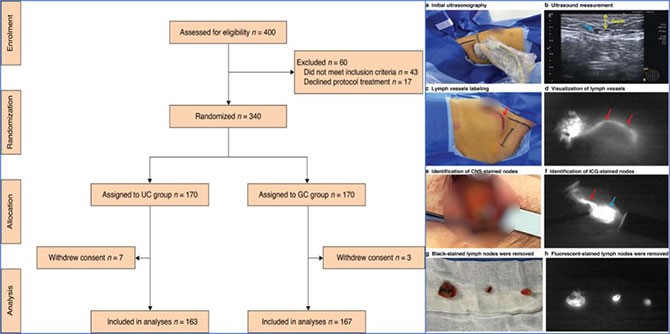
This study is the first study where the intraoperative ultrasound-assisted technique was used in sentinel lymph node biopsy surgery, and the first randomized controlled study where the carbon nanoparticle suspension independently developed by our country was used in sentinel lymph node tracer. The study has reduced the difficulty of looking for stained lymph nodes during surgery by using an intraoperative ultrasound-assisted technique and broken through the clinical bottleneck that only dual tracers can achieve a high detection rate and lower false negative rate. It has also provided preliminary data support for further application in patients after neoadjuvant therapy.
Zhang Liulu