

Pancreatic cancer is a highly malignant tumor in the digestive system, and is expected to become the second most lethal tumor in the next decade with its incidence rate rising year by year. Lymphatic metastasis is one of the most important metastasis pathways of pancreatic cancer. Once it occurs, the 5-year survival rate of patients will drop sharply by more than 20%. Our previous research has confirmed that as the most common mutation type of pancreatic cancer, mutations of the kirsten rat sarcoma viral oncogene (KRAS) play an important role in the lymphatic metastasis of pancreatic cancer by mediating extracellular vesicles to reshape the lymphatic system in the tumor microenvironment.
Prof. Chen Rufu’s team carried out a systematic study on the accumulation of abnormal epigenetic events in pancreatic cancer with KRAS mutations, and the related findings were published in Cancer Research (impact factor: 13.312) under the title of “Mutant KRAS mediates circARFGEF2 biogenesis to promote lymphatic metastasis of pancreatic ductal adenocarcinoma”, which further clarify the core molecular mechanism of the KRAS mutation-mediated abnormal cyclization of ribonucleic acids (RNAs) leading to the accumulation of circular RNAs (circRNAs) and driving the lymphatic metastasis of pancreatic cancer, and provide a new strategy for blocking the accumulation of abnormal epigenetic events driven by KRAS mutations and developing new interventions for the lymphatic metastasis of pancreatic cancer.

This study explores the key molecular mechanism of the lymphatic metastasis of pancreatic cancer from the perspective of the accumulation of abnormal epigenetic events based on mutations of key tumor driving genes, clarifies the precise regulatory mechanism of the KRASG12D mutation (the amino acid at Site 12 of the KRAS mutates from glycine to aspartic acid) mediated abnormal circRNA synthesis and the regulation of the lymphatic metastasis of pancreatic cancer by signal accumulation, and preliminarily confirms that the generation of an abnormal circRNA signal in pancreatic cancer with the targeted KRASG12D mutation in the body can inhibit the lymphatic metastasis of pancreatic cancer. In addition, the team’s previous research has confirmed that KRAS mutations can also promote the lymphatic metastasis of pancreatic cancer by extracellular regulation-mediated tumor microenvironment reshaping. On this basis, developing means to block KRAS mutation-mediated intracellular and extracellular abnormal signals jointly may become an important strategy to intervene in pancreatic cancer metastasis.
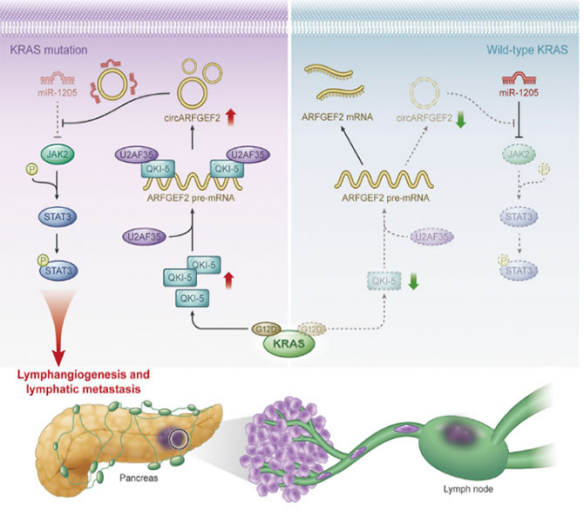
Prof. Chen Rufu’s team has been committed to mechanism and clinical research in pancreatic cancer metastasis for a long time. They have carried out extensive research on tumor microenvironment-mediated neural invasion and tumor metastasis of pancreatic cancer, and the related findings have been published in The Journal of Clinical Investigation, Molecular Cancer, Oncogene, Journal of Experimental & Clinical Cancer Research, etc. This study fills up the gap in the accumulation and regulation of abnormal signals of tumors. The above serial studies jointly analyze the tumor-nerve-immune regulation network in pancreatic cancer, and are expected to realize the transformation from single-point mechanism breakthroughs to the establishment of a multi-target, multi-channel comprehensive clinical treatment system.
Guangdong Provincial People’s Hospital is the first completing unit of the article, postdoctoral fellow Kong Yao, Dr. Luo Yuming, Dr. Zheng Shangyou, and Dr. Yang Jiabin from the Pancreas Center, Guangdong Provincial People’s Hospital are the joint first authors, and Prof. Chen Rufu is the corresponding author.
Pancreas Center
Updated: July 17, 2023