

Stage III non-small cell lung cancer (NSCLC) is a highly heterogeneous group of tumors with complex treatment options. Some patients can be treated surgically, while others are deemed unresectable for various reasons. For unresectable stage III NSCLC, the currently recommended treatment regimen is concurrent chemoradiotherapy followed by consolidation immunotherapy. However, only about 70% of patients have the opportunity to proceed to consolidation immunotherapy after chemoradiotherapy. Would it be possible to achieve better treatment outcomes if immunotherapy were used before chemoradiotherapy to convert these unresectable patients into resectable ones?
To address this question, the team led by Prof. Wu Yilong from Guangdong Provincial People’s Hospital and Guangdong Lung Cancer Institute proposed a groundbreaking concept: converting “unresectable” to “resectable.” They conducted a proof-of-concept study called the TRAILBLAZER study to explore the efficacy and safety of “early” immune induction therapy in these patients. This important research finding was published in full in the journal Cancer Cell on June 20, 2024. The journal enjoys a high reputation in academia with an impact factor of 50.3.

This study used SHR-1701, an immunotherapy drug independently developed in China, and enrolled 107 patients with unresectable stage III NSCLC, who were grouped according to different conditions. After completing 3 cycles of SHR-1701 combined with chemotherapy induction treatment, all patients underwent multidisciplinary team (MDT) consultation to determine the next step of treatment. For patients assessed as resectable by MDT, surgical treatment was performed; if still assessed as unresectable, those would enter the Department of Radiotherapy for radical chemoradiotherapy. After completing local treatment, all patients started 16 cycles of SHR-1701 consolidation therapy, followed by a follow-up phase.
As of September 2023, the median follow-up time reached 22.2 months. The study results showed that after induction therapy, 25% of originally unresectable stage III NSCLC patients achieved surgical conditions, and all patients achieved R0 resection. Among them, 12 patients (44%) achieved major pathological response (MPR), 7 patients (26%) achieved pathological complete response (pCR), and the 18-month event-free survival rate was 74.1%. For patients receiving radiotherapy, the 18-month event-free survival rate was 57.3%. In other words, SHR-1701 combined with chemotherapy induction treatment converted about 1/4 of originally unresectable patients to resectable treatment, and patients who received surgery showed significantly better outcomes than those who received chemoradiotherapy.
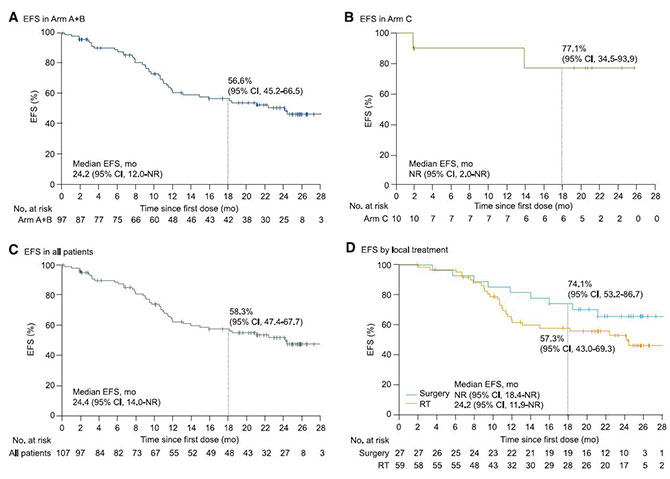
This groundbreaking study showed that after receiving an immunotherapy-based induction treatment regimen, some unresectable stage III NSCLC patients can be converted to resectable status. These patients may achieve a cure after surgical treatment, revealing advantages over the chemoradiotherapy followed by consolidation immunotherapy model. This cleverly designed study provides a new treatment model for unresectable stage III NSCLC.

TRAILBLAZER Research Team
Why can a phase II clinical trial be published in a top international journal? “This is an exploratory phase II clinical trial, not a large phase III clinical trial. The more important value of this study lies in exploring an innovative concept - convertibility, which brings a revolutionary change in treatment model for mid-stage unresectable patients,” said Prof. Zhou Qing, the first author.
“At present, this is still a proof-of-concept study. In other words, this concept is unprecedented, and the study is to prove whether this concept can be established,” said Prof. Wu Yilong. “To make the results of this study truly become a universal treatment model, there is still a long way to go. Currently, the team is preparing to launch a larger-scale study to confirm this concept, and Prof. Pan Yi from the Department of Radiotherapy is also leading a related real-world study. If these two studies are successfully completed and proven effective, it is expected to change clinical strategies and become a new treatment model used worldwide.”
Ke E’e, Zhou Qing
Updated: 2 July, 2024