

Distant metastasis is regarded as one of the primary causes of poor prognoses and deaths in colorectal cancer (CRC), and the liver is one of the most common organs for distant metastasis in CRC. The failure of first-line chemotherapy is recognized as one of the important causes of therapeutic difficulties in CRC liver metastasis (CRCLM). Finding new and effective therapeutic targets for CRC chemoresistance and liver metastasis, and exploring effective therapeutic strategies for CRCLM patients are of great clinical significance.
Focusing on this critical clinical issue, Prof. Yao Xueqing’s team from the Department of Gastrointestinal Surgery, Guangdong Provincial People’s Hospital has identified key targets for CRC chemoresistance and liver metastasis for the first time, and established a targeted tumor therapy using engineered primary tumor-derived exosomes loaded with siRNA, providing a new strategy for overcoming CRC oxaliplatin chemoresistance and liver metastasis. The relevant research findings were published in ACS NANO, a top-tier journal in CAS Q1 (DOI:10.1021/acsnano.3c00668, impact factor: 18.024) under the title of “Delivery of Engineered Primary Tumor-Derived Exosomes Effectively Suppressed the Colorectal Cancer Chemoresistance and Life Metastasis”, which was selected as the cover article of this issue. Doctoral student Huang Chengzhi, master student Zhou Yue, and deputy chief physician Feng Xingyu from the Department of Gastrointestinal Surgery, Guangdong Provincial People’s Hospital are the first authors of this article; Prof. Yao Xueqing, Prof. Li Yong, and Prof. Wang Junjiang from the Department of Gastrointestinal Surgery, Guangdong Provincial People’s Hospital are the corresponding authors of this article.


In this study, the key target CCDC80 (Coil-coil Domain-Containing Protein 8) for CRC chemoresistance and liver metastasis was identified through RNA sequencing combined with bioinformatic analysis, and the biological function of CCDC80 was validated through in vitro and in vivo experiments. It was found that inhibiting CCDC80 expression could enhance the sensitivity of cell lines and tumor-bearing mice to oxaliplatin treatment significantly. On this basis, this study built engineered primary tumor-derived exosomes loaded with siRNA with better biocompatibility for targeted tumor treatment.
“Exosomes are vesicles secreted by most mammalian cells, predominantly with particle sizes of 40-150nm. Exosomes are regarded as one of the key messengers for intercellular information communication. They can encapsulate non-coding RNAs, proteins, cytokines, secretion products, etc. and transfer them to receptor cells for intercellular information communication. As exosomes are endogenous secretory products of mammals, they have high biocompatibility and low immunogenicity, and thus have great potential of application to the delivery of nucleic acids or chemotherapeutic drugs.”
In this study, primary cells were extracted from tumor tissues of CRC patients, and exosomes were secreted, purified, and modified using bioengineering technology to enhance their drug carrying and targeting capacities, slowing down exosome aggregation and protein adsorption, and reducing side effects such as renal exosome clearance. Through in vitro and in vivo experiments, it was found that tumor-derived exosomes were more inclined to aggregate at tumor sites, indicating that engineered exosomes had a certain “homing” ability and the function of targeting tumor tissues. The therapeutic effect was validated in a patient-derived xenograft (PDX) model, a CRCLM mouse model, and a patient-derived organoid xenograft (PDOX) model.
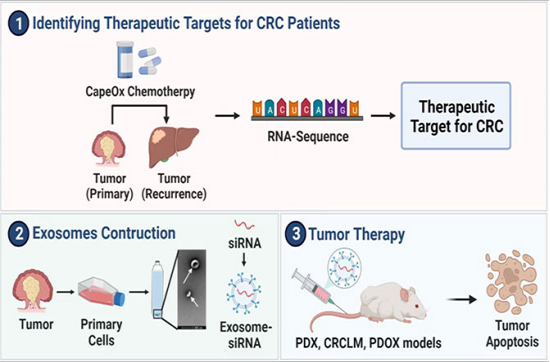
The PDOX mouse model simulated CRCLM. Under the conventional therapy of surgery plus chemotherapy, the survival of mice was unsatisfactory. However, tumor-bearing mice that underwent partial hepatectomy and received the co-administration of engineered exosomes and oxaliplatin showed the best overall survival. The research findings suggest that the use of engineered exosomes combined with oxaliplatin chemotherapy can improve the efficacy of chemotherapy significantly, reverse chemoresistance in CRC, and improve the survival prognosis of CRCLM.
Therefore, this study has developed a brand-new therapy for CRCLM using clinical samples, primary tumor-derived exosomes, and an approach closest to clinical treatment based on clinical practice.
Reference: Delivery of Engineered Primary Tumor-Derived Exosomes Effectively Suppressed the Colorectal Cancer Chemoresistance and Liver Metastasis. ACS NANO. 2023 May 5. 10.1021/acsnano.3c00668
Huang Chengzhi
Updated: May 23, 2023