

Colorectal cancer (CRC) is a clinically common malignant tumor of the digestive tract, with its incidence and mortality rates ranking third and second among all malignant tumors, respectively, posing a serious threat to human health. Tumor metastasis is often the primary cause of patient death, and the liver is the most common organ for CRC metastasis. Analyzing differences in microenvironment between primary and liver metastatic foci of CRC can help clarify the mechanism of tumor metastasis and develop new clinical therapeutic targets.
For the above issue, Prof. Lian Zhexiong’s team from Guangdong Provincial People’s Hospital and Prof. Cao Jie’s team from Guangzhou First People’s Hospital jointly published a study titled “Single-cell and spatial transcriptome analysis reveals the cellular heterogeneity of liver metastatic colorectal cancer” in Science Advances on June 16, 2023. This study reveals the differences in tissue microenvironment between primary and liver metastatic foci of CRC at the single-cell and spatial levels, especially the differences in fibroblast subgroups of primary and liver metastatic foci, explores interactive relationships among different components, and is of great guiding significance for further screening new therapeutic targets.
First, the author collected primary foci, liver metastatic foci, para-carcinoma controls in the intestinal tract and liver, and peripheral blood from CRC patients, sorted out CD45+ immune cells and CD45 non-immune cells, and identified 23 non-immune cell subgroups and 41 immune cell subgroups on the BD Rhapsody single cell sequencing platform (Figure 1).

Figure 1
By analyzing the immune cell subgroups, the authors found that CD8+T cells responsive to highly expressed CXCL13 tumors were enriched in cancer tissues, and their also highly expressed NOTCH signaling pathway-specific transcription factor RBPJ suggested that they might be regulated by the NOTCH signaling pathway. In addition, there was a group of cancer-associated fibroblasts (CAFs) with specifically highly expressed F3 in primary foci of CRC, which were almost nonexistent in liver metastatic foci. A gene difference analysis showed that F3+CAF could generate a number of tumor promoting factors, such as VEGFA, NRG1, HGF, GDF15, AREG, and BMP2. The higher the proportion of this cell group was, the worse patient prognoses would be. Unlike primary foci, a group of CAFs with highly expressed MCAM was enriched in the liver, and highly expressed the ligand JAG1 of the NOTCH signaling pathway. An interaction analysis suggested that the MCAM+CAF subgroup might promote the generation of tumor-responsive CD8+T cells through the JAG1-NOTCH1 signaling pathway. Finally, the authors further collected paraffin tissues from primary and liver metastatic foci of CRC, identified different regions of these tissues through the 10X spatial transcriptomics platform, and further validated the above interactive relationships based on spatial transcriptomics information (Figure 2).
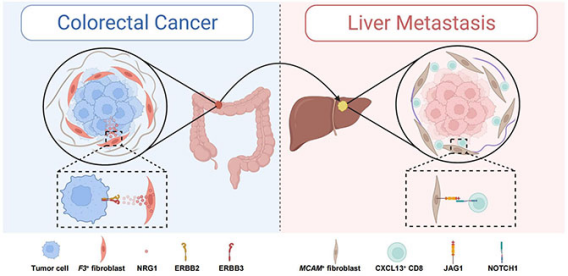
Figure 2
In addition, this article reveals the heterogeneity of tumor cells, as CRC tumor cells inherit part of the differentiation ability of normal intestinal epithelial cells and generate tumor cell subgroups with different gene expression characteristics. Such heterogeneity of tumor cells may be an important reason why some anti-tumor therapies fail. Identifying the composition of tumor cells by single-cell sequencing may show the way for the individualized precise treatment of cancer patients.
The differences between primary and hepatic metastatic foci of CRC revealed in this article, especially the differences in stromal microenvironment, can explain the drug resistance of tumors, and the differences in treatment responses between primary and metastatic foci to some extent. Targeting the stromal microenvironment to intervene in tumor progression may become an important anti-tumor strategy in the future.
Postdoctoral fellows Wang Fei and Long Jie from Guangdong Provincial People’s Hospital are the joint first authors of this article. Prof. Lian Zhexiong, Prof. Ma Haiqing, and associate research fellow Zhao Zhibin from Guangdong Provincial People’s Hospital, and Prof. Cao Jie from Guangzhou First People’s Hospital are the joint corresponding authors of this article. In addition, this study received strong support from Prof. Yao Xueqing from Guangdong Provincial People’s Hospital.
Original link: https://www.science.org/doi/10.1126/sciadv.adf5464
Wang Fei
Updated: June 29, 2023