

Prof. Wang Lijuan’s team from the Department of Neurology of our hospital has made a significant breakthrough in the research on cognitive impairment mechanisms in α-synucleinopathies, and the research findings were published in the world-renowned journal Advanced Science (impact factor: 17.521) on June 29, 2023.
The paper titled “Cerebral microvascular damage induced by Lag3-dependent α-synuclein fibril endocytosis exacerbates cognitive impairment in a mouse model of α-synucleinopathies” delves into and demonstrates a common microvascular pathological mechanism of cognitive impairment in α-synucleinopathies.

α-synucleinopathies are a collective term for a class of neurodegenerative diseases, including Parkinson’s disease (PD), dementia with Lewy bodies (DLB), multiple system atrophy (MSA), the Lewy body variant of Alzheimer’s disease (LBV-AD), and pure autonomic failure (PAF). They are characterized in that pathological α-synuclein (α-Syn) misfolds and abnormally aggregates in damaged neurons, and gradually forms insoluble α-syn inclusion bodies (the main component of Lewy bodies) in neurons. This “pathological α-Syn” can intrude into the central and peripheral nervous systems, and result in a wide range of motor and non-motor clinical symptoms. The chronic degeneration and death of neurons during disease occurrence and development are often not the direct cause of sufferer death, while complications in the late stage of diseases ultimately lead to sufferer death. In particular, cognitive impairment is a main non-motor symptom of α-synucleinopathies and one of the most common causes of death, and causes sufferers to lose their self-care abilities in the late stages of diseases.
Looking for the key causes of cognitive impairment in α-synucleinopathies is crucial. Since 2017, with the improvement of clinical laboratory technology, an increasing number of clinical studies have detected increased plasma α-Syn levels in sufferers with various α-synucleinopathies. And the higher the plasma α-Syn level is, the more severe the cognitive impairment of sufferers will be. It is worth studying whether there is a common vascular pathological mechanism for cognitive impairment in α-synucleinopathies!
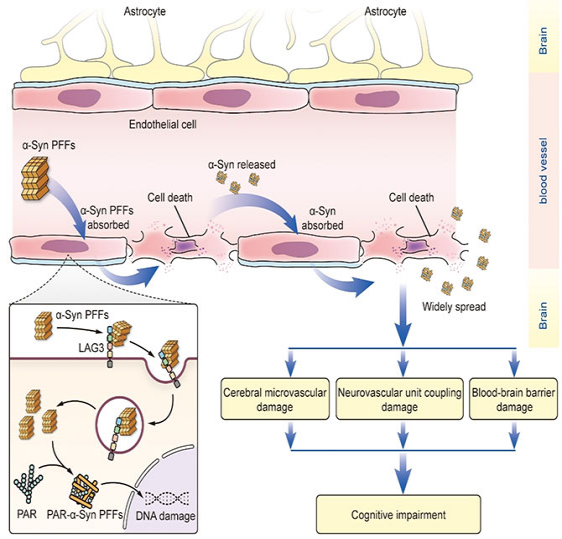
This study proves in detail for the first time that α-Syn fibers enter cerebral microvascular endothelial cells (BMVECs) through lymphocyte-activation gene 3 (Lag3)-dependent endocytosis, resulting in cognitive impairment independent of nerve damage. Prof. Wang Lijuan’s team has established a stable cognitive impairment model for α-synucleinopathies: In a mouse model, mice with α-Syn preformed fibers (PFFs) injected into the substantia nigra pars compacta, hippocampus, and cerebral cortex on one side jointly showed impaired spatial learning and memory abilities 6 months after injection, and this decline in cognitive abilities was associated with cerebral microvascular damage. PFFs can form insoluble α-Syn in BMVECs through Lag3-dependent endocytosis, resulting in a series of cerebral microvascular damage effects, including cell death driven by poly ADP-ribose (PAR), reduced expression of tight junction proteins in BMVECs, disruption of the blood-brain barrier, disruption of neurovascular unit coupling, and disruption of the cerebral microvascular network. These effects may be an important common pathological mechanism that exacerbates cognitive impairment in α-synucleinopathies. The in-vitro knockout of Lag3 can prevent PFFs from entering BMVECs, thereby reducing the above effects induced by PFFs. The loss of endothelial cell specific Lag3 in the body can reverse cerebral microvascular damage and cognitive impairment induced by PFFs.
Therefore, this study provides a key evidence for a common microvascular pathological mechanism of cognitive impairment in α-synucleinopathies, and reveals the great significance of blocking the diffusion of α-Syn fibers into BMVECs by targeting Lag3 for improving cognitive function.
More importantly, in clinical practice, sufferers of α-synucleinopathies with cognitive impairment are often combined with cerebral (small) vascular diseases, and some sufferers of cerebral (small) vascular diseases are also combined with α-synucleinopathies. Pathological α-Syn’s own neuronal damage and extensive cerebral (small) vascular damage can both cause cognitive impairment, making it difficult for neurologists to distinguish between the exact causes of cognitive impairment in sufferers with these two types of diseases at diagnosis. Tracing and sorting out the impacts of these two different pathological factors on the cognitive function is a key concern for neurologists. In the past, etiological research on these two types of diseases often focused on their own pathological development, such as nerve damage arising from pathological α-Syn changes in α-synucleinopathies, and vascular damage caused by cerebral stroke, chronic (ischemic) hypoperfusion, peripheral factors (hypertension, diabetes, hyperlipidemia, infection, etc.) in cerebral (small) vascular diseases. It is not clear whether these two types of diseases have a common pathological origin. This study suggests that these two types of diseases are not “completely distinct”! Pathological α-Syn causes both neuronal damage and cerebral microvascular damage itself, where the latter independently damages the cognitive function of sufferers. This study also provides deeper insights into the etiology of cognitive impairment in sufferers with these two major types of diseases, and has profound clinical significance!
The Department of Neurology, Guangdong Provincial People’s Hospital is the first completing unit of this article, postdoctoral fellow Zhang Qingxi is the first author, Director Wang Lijuan is the corresponding author, and deputy chief physician Nie Kun is a joint corresponding author. In addition, doctoral student Duan Qingrui and physician Gao Yuyuan from the Department of Neurology are the joint first authors.
Zhang Qingxi, the first author of this study, graduated from Southern Medical University as a postdoctoral researcher and studied under Prof. Wang Lijuan. She has been studying molecular mechanisms of neurodegenerative diseases and cerebral microvascular diseases, published a series of research findings on vascular mechanisms of cognitive impairment in α-synucleinopathies (published in Advanced Science), the abnormal deposition mechanism of mitochondrial pathological α-Syn (published in Int J Biochem Cell Biol), research on α-Syn pathological model simulation and treatment for Parkinson’s disease (published in such journals as Front Mol Neurosci and Human Gene Therapy), and other related research directions, and led some general projects of the China Postdoctoral Science Foundation and some youth development projects under the National Natural Science Foundation in our hospital as the project leader.
Department of Neurology
Updated: July 13, 2023